Group PD Dr. Schoppmeier
Tribolium early segmentation and oogenesis
Introduction
We are studying the evolution of the segmentation process using the red flour beetle Tribolium castaneum as model. In the last decade Tribolium has become a model system for comparative developmental biology. Many genetic and molecular techniques, including chemical and insertional mutagenesis, systemic RNA interference, and germ-line transformation are available for Tribolium.
The molecular mechanisms underlying segmentation have been studied in detail in the fruit fly Drosophila, where segmentation is controlled by a hierarchical cascade of genes that depends on the formation of long- and short-range gradients of transcription factors. In Drosophila those gradients can easily form in the early syncytial blastoderm embryo, as this embryo is not cellularised yet (long-germ development). In Tribolium, as in most insects, the posterior segments are added from a posteriorly located growth zone (short-germ development), suggesting that formation of these segments may rely on a different mechanism.
Blastodermal patterning
How is the Tribolium embryo patterned and which spatial cues are involved in setting up the anterior to posterior axis? To answer these questions we are analyzing the maternal system of Tribolium by functional and transgenic essays. Interestingly most maternal factors that have crucial roles in patterning the Drosophila blastoderm are either not present in Tribolium (e.g. bicoid) or have different roles during early segmentation (e.g. orthodenticle, caudal, nanos, pumilio), indicating that blastoderm patterning in Tribolium involves additional genes (e.g. Mex-3). To identify additional, possibly short-germ specific genes, we make use of genome-wide RNA interference screens and Next generation sequencing (NGS) technologies, including transcriptome analysis (RNA-seq).
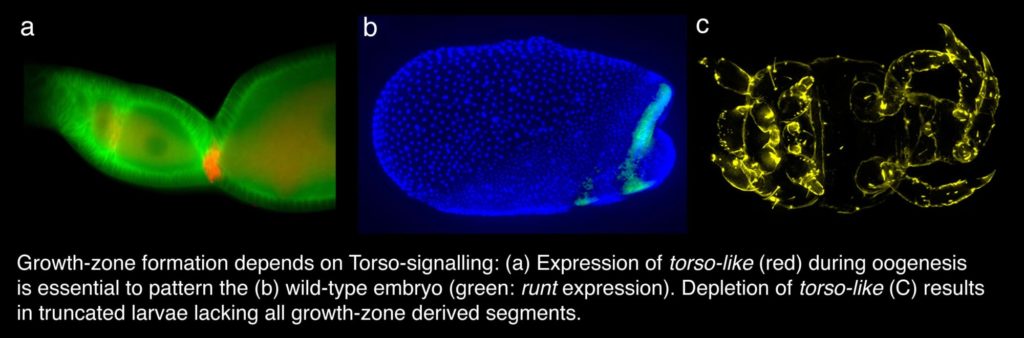
The formation of the growth-zone
How is the elongation of the body axis initiated? We could show that the formation of the growth-zone depends on Torso-signalling, which is already active during oogenesis. Depletion of either Torso or torso-like results in larvae that lack all growth-zone derived segments. In addition the serosa is severely reduced and the head region is enlarged. Thus, in a short-germ embryo Torso-signaling is required – like in Drosophila – for patterning both the anterior and the posterior region of the embryo. Due to the difference in the anlagen-plan of short and long germ embryos, however, different tissues and different processes depend on the Torso-signaling.
Axis formation during oogenesis
In Drosophila axis formation is the direct consequence of symmetry-breaking events that take place throughout oogenesis. In the polytrophic meroistic mode of Drosophila oogenesis each growing oocyte is supported by an individual subset of clonal nurse cells. Tribolium, however, represents telotrophic oogenesis.
In each ovariole the nurse cells of all cysts are kept in at the terminal region (Tropharium) and the oocytes will be nourished by elongating nutritive cords connecting the growing oocytes with the Tropharium. To get insights into the symmetry-breaking events during telotrophic oogenesis, we have started to analyze the molecular mechanisms controlling Tribolium oogenesis. This includes oocyte selection and encapsulation, follicle cell patterning, and the regulation of different stem cell populations.
DFG Research Unit FOR 1234 iBeetle: Functional Genomics of Insect Development and Metamorphosis
The red flour beetle Tribolium castaneum has developed into a major insect model system second only to Drosophila melanogaster. The powerful reverse genetics and the available genomic sequence allow us to conduct an unbiased genome-wide RNA interference screen targeting diverse biological questions. With this DFG funded “iBeetle screen” we intend to overcome the currently prevailing, candidate gene approach in arthropods outside of Drosophila, and aim at further transforming Tribolium into a powerful primary model system suited for basic research in insect development, evolution, physiology, and pest control. ibeetle.uni-göttingen.de
Lab alumni
| Name | Position | Period | Current Position |
| Fabian Pridöhl | PhD student | 2012-2016 | Postdoc, Institut für Entwicklungsbiologie, Universität Köln |
| Denise Mackrodt | PhD student | 2012-2015 | Fachredakteurin, Helmholtz Zentrum München |
| Nadi Ströhlein | PhD student | 2009-2012 | Clinical Research Associate, Novartis Pharma |
| Daniel Bäumer | PhD student | 2007-2011 | Medical Advisor, Novartis Pharma |
| Christian Schmitt-Engel | PhD student | 2006-2010 | Referent, Graduiertenzentrum der FAU |
